110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

G250 抗原靶向的载药纳米泡与超声靶向的纳米泡破坏技术相结合:针对肾细胞癌的一种潜在的新型治疗方法
Authors Yu Z, Wang Y, Xu D, Zhu L, Hu M, Liu Q, Lan W, Jiang J, Wang L
Received 14 September 2019
Accepted for publication 16 December 2019
Published 8 January 2020 Volume 2020:15 Pages 81—95
DOI https://doi.org/10.2147/IJN.S230879
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Purpose: We intended to design G250 antigen-targeting temsirolimus-loaded nanobubbles (G250-TNBs) based on the targeted drug delivery system and to combine G250-TNBs with ultrasound targeted nanobubble destruction (UTND) to achieve a synergistic treatment for renal cell carcinoma (RCC).
Methods: The filming-rehydration method was combined with mechanical shock and electrostatic interactions to prepare temsirolimus-loaded nanobubbles (TNBs). G250-TNBs were prepared by attaching anti-G250 nanobodies to the surface of TNBs using the biotin-streptavidin-bridge method. The ability of G250-TNBs to target the G250 antigen of RCC cells and the synergistic efficacy of G250-TNBs and UTND in the treatment of RCC were assessed.
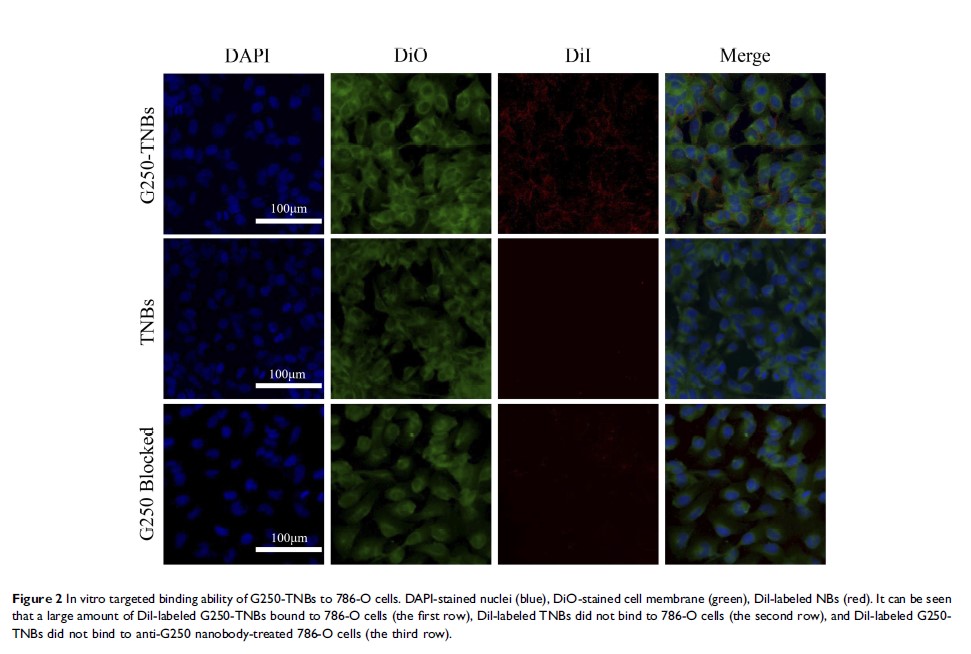
Results: The average diameter of the prepared G250-TNBs was 368.7 ± 43.4 nm, the encapsulation efficiency was 68.59% ± 5.43%, and the loading efficiency was 5.23% ± 0.91%. In vitro experiments showed that the affinity of G250-TNBs to the human RCC 786-O cells was significantly higher than that of TNBs (P <0.05), and the inhibitory effect on 786-O cell proliferation and the induction of 786-O cell apoptosis was significantly enhanced in the group treated with G250-TNBs and UTND (G250-TNBs+ UTND group) compared with the other groups (P <0.05). In a nude mouse xenograft model, compared with TNBs, G250-TNBs could target the transplanted tumors and thus significantly enhance the ultrasound imaging of the tumors. Compared with all other groups, the G250-TNBs+UTND group exhibited a significantly lower tumor volume, a higher tumor growth inhibition rate, and a higher apoptosis index (P <0.05).
Conclusion: The combined G250-TNBs and UTND treatment can deliver anti-tumor drugs to local areas of RCC, increase the local effective drug concentration, and enhance anti-tumor efficacy, thus providing a potential novel method for targeted therapy of RCC.
Keywords: nanobubble, G250 antigen, nanobody, targeted drug delivery system, renal cell carcinoma, temsirolimus