110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

哺乳期乳腺炎使用或不使用抗生素的脓疡引流:一项随机对照试验的研究方案
Authors Luo J, Long T, Cai Y, Teng Y, Fan Z, Liang Z, Zhu C, Ma H, Li G
Received 28 June 2019
Accepted for publication 24 December 2019
Published 21 January 2020 Volume 2020:13 Pages 183—190
DOI https://doi.org/10.2147/IDR.S221037
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens
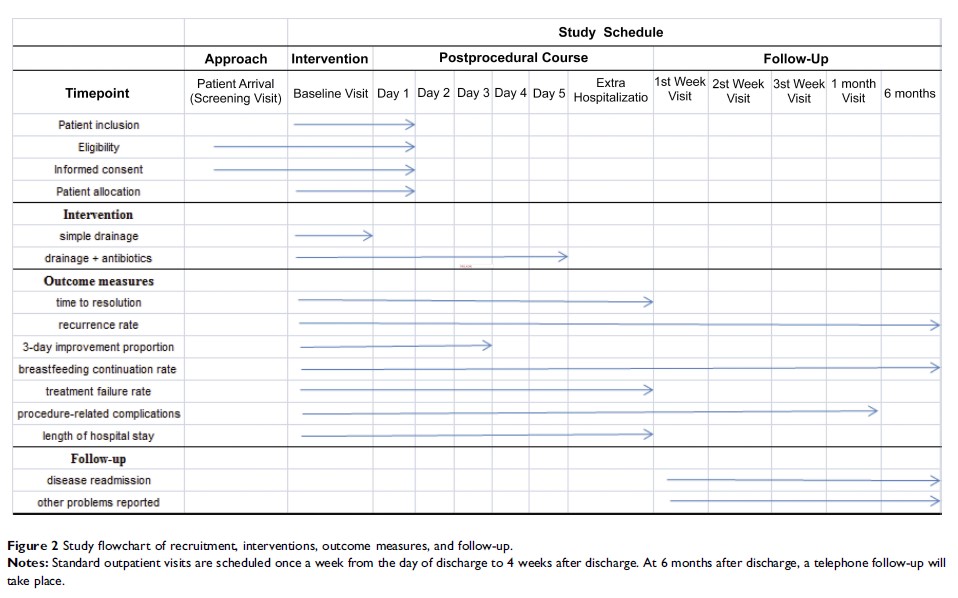
Background: Lactational breast abscess, a complication from lactational mastitis, is a common cause of breastfeeding discontinuation. No consensus has been reached regarding the necessity of antibiotics in this disease. The purpose of this trial is to determine if surgical drainage is non-inferior to drainage together with a standard course of antibiotics, in the treatment of lactational breast abscess.
Methods: Breastfeeding females with breast abscess from 18 to 50 years old are eligible for study inclusion. An expected number of 306 patients will be randomly allocated in parallel to the intervention arm (simple drainage without antibiotics) or the control arm (abscess drainage with standard 5-day-course of antibiotics). The primary outcomes include the time to resolution of breast abscess and disease recurrence rate. Secondary outcomes of interests are 3-day-improvement proportion, rate of continuing breastfeeding, treatment failure rate, procedural-related complications, and length of hospital stay. An expected non-inferiority margin for the difference in the primary outcome of interest is set at 1 day, on the basis of a one-sided 97.5% confidence interval.
Discussion: This trial will provide first-hand evidence on whether simple abscess drainage is non-inferior to drainage together with a standard course of antibiotics, in lactational mothers with breast abscess. The indication of antibiotic prophylaxis could be revised if non-inferiority is set up, and guidelines for lactational breast abscess require amendments correspondingly.
Trial Registration: This study has been registered in the Chinese Clinical Trial Registry, and the trial registration number is ChiCTR1900024008.
Keywords: lactational breast abscess, antibiotics, drainage, randomized controlled trial, study protocol