110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

甲磺酸阿帕替尼用于治疗在接受标准治疗后出现发展的晚期非鳞状非小细胞肺癌患者的临床疗效和安全性以及 KDR 基因多态性分析
Authors Song ZZ, Zhao LF, Zuo J, Fan ZS, Wang L, Wang YD
Received 13 July 2019
Accepted for publication 17 December 2019
Published 21 January 2020 Volume 2020:13 Pages 603—613
DOI https://doi.org/10.2147/OTT.S222985
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 3
Editor who approved publication: Dr Yao Dai
Purpose: This study investigated the clinical outcomes and safety of apatinib mesylate in the treatment of advanced non-squamous non-small cell lung cancer (NSCLC) in patients who progressed after standard therapy, and analyzed the kinase insert domain receptor (KDR ) gene polymorphism.
Methods: A total of 135 patients with advanced non-squamous NSCLC who received apatinib mesylate were included. Objective response rates were evaluated. Subsequently, progression-free survival (PFS) and overall survival (OS) were assessed and safety data were recorded. Additionally, peripheral blood and biopsy cancer tissue specimens were collected from the patients with NSCLC for the genotyping of the genetic polymorphism and mRNA expression of the KDR gene, respectively. Analysis on the association between genotypes and prognosis was conducted.
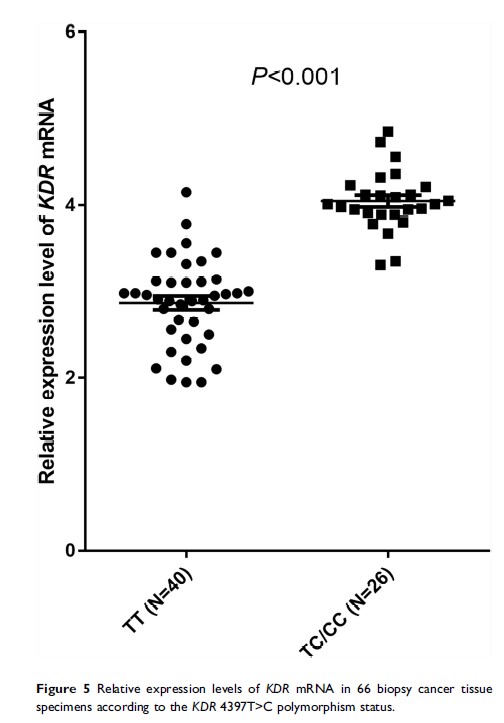
Results: The objective response rate of the 135 patients with NSCLC was 18.52%, disease control rate was 65.19%, median PFS was 3.95 months, and median OS was 10.05 months. Regarding the KDR gene polymorphism analysis, the distribution of the 4397T>C polymorphism genotypes was in accordance with the Hardy–Weinberg Equilibrium (P =0.868). Moreover, the prognosis analysis indicated that the median PFS of patients with the CC/TC and TT genotypes was 2.80 and 4.80 months, respectively (P =0.002). Furthermore, the median OS of patients with the two genotypes was 9.10 and 10.56 months, respectively (P =0.041). The multivariate Cox regression analysis showed that the TC/CC genotypes were an independent factor for PFS (odds ratio: 1.72, P =0.009). There was no correlation between the polymorphism and adverse reactions. Additionally, the mRNA expression analysis suggested that the mRNA levels of KDR in cancer tissues were significantly different between the TT and TC/CC genotypes (P < 0.001).
Conclusion: The clinical outcomes of treatment with apatinib mesylate for advanced non-squamous NSCLC in patients who progressed after standard therapy may be influenced by the KDR 4397T>C polymorphism through mediation of the mRNA expression of KDR .
Keywords: non-small cell lung cancer, apatinib mesylate, kinase insert domain receptor , polymorphism, clinical outcomes, safety