110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

阿帕替尼用于治疗复发性或难治性弥漫性大 B 细胞淋巴瘤患者:阶段 II、单臂开放标签的前瞻性研究
Authors Ma X, Li L, Zhang L, Fu X, Li X, Wang X, Wu J, Sun Z, Zhang X, Feng X, Chang Y, Zhou Z, Nan F, Zhang J, Li Z, Zhang M
Received 16 August 2019
Accepted for publication 17 December 2019
Published 22 January 2020 Volume 2020:14 Pages 275—284
DOI https://doi.org/10.2147/DDDT.S227477
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Purpose: Treatment options for relapsed or refractory diffuse large B-cell lymphoma (RR DLBCL) represent an unmet medical need. Apatinib is a new oral tyrosine kinase inhibitor mainly targeting vascular endothelial growth factor receptor-2 (VEGFR-2) to inhibit tumour angiogenesis. In the present study, we evaluated the efficacy and safety of apatinib for patients with RR DLBCL.
Patients and Methods: In this phase II, open-label, single-arm, prospective study, we enrolled patients aged 14– 70 years with treatment failure of at least two chemotherapeutic regimens using Simon’s two-stage design. All patients were administered apatinib at an initial dose of 500 mg on a 4-week cycle at home and visited the outpatient clinic every two cycles to evaluate efficacy and to record adverse events. We considered objective response rate (ORR) as the primary end point, and progression-free survival (PFS), and overall survival (OS) plus duration of response (DoR) as the secondary end point. (This trial was registered at ClinicalTrials.gov, identifier: NCT03376958.).
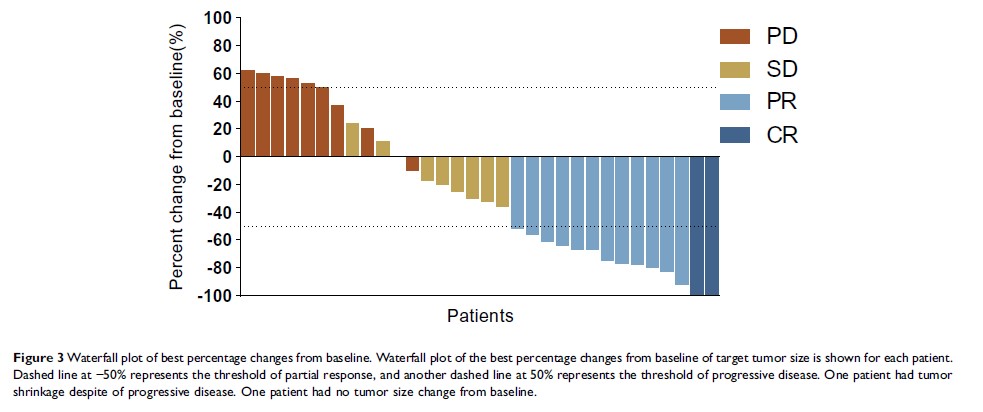
Results: From January 2017 to February 2019, we screened 35 patients and enrolled 32 eligible patients. At the cutoff point (April 2019), we noted 2 (6.3%) complete responses, 12 (37.5%) partial responses, and 9 (28.1%) stable diseases, attributing to an ORR of 43.8% and a disease control rate of 71.9%. The median PFS and OS were 6.9 (95% confidence interval [CI], 5.8– 7.9) and 7.9 months (95% CI, 7.0– 8.7), respectively. The median DoR was 5.0 months (95% CI, 3.5– 6.5) for patients who achieved PR. The most common grade 3– 4 adverse events (AE) were hypertension (12.6%), hand–foot syndrome (9.4%), and leucopenia (6.3%). No apatinib-related deaths were noted.
Conclusion: Home administration of apatinib shows promising efficacy and manageable AEs in patients with RR DLBCL.
Keywords: apatinib, relapsed or refractory diffuse large B-cell lymphoma, VEGFR-2, efficacy, safety