110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

藤黄酸纳米颗粒复合热敏水凝胶和肿瘤穿透肽 iRGD 对胃癌的抗肿瘤活性
Authors Zhang D, Chu Y, Qian H, Qian L, Shao J, Xu Q, Yu L, Li R, Zhang Q, Wu F, Liu B, Liu Q
Received 18 September 2019
Accepted for publication 2 January 2020
Published 31 January 2020 Volume 2020:15 Pages 735—747
DOI https://doi.org/10.2147/IJN.S231448
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 3
Editor who approved publication: Dr Mian Wang
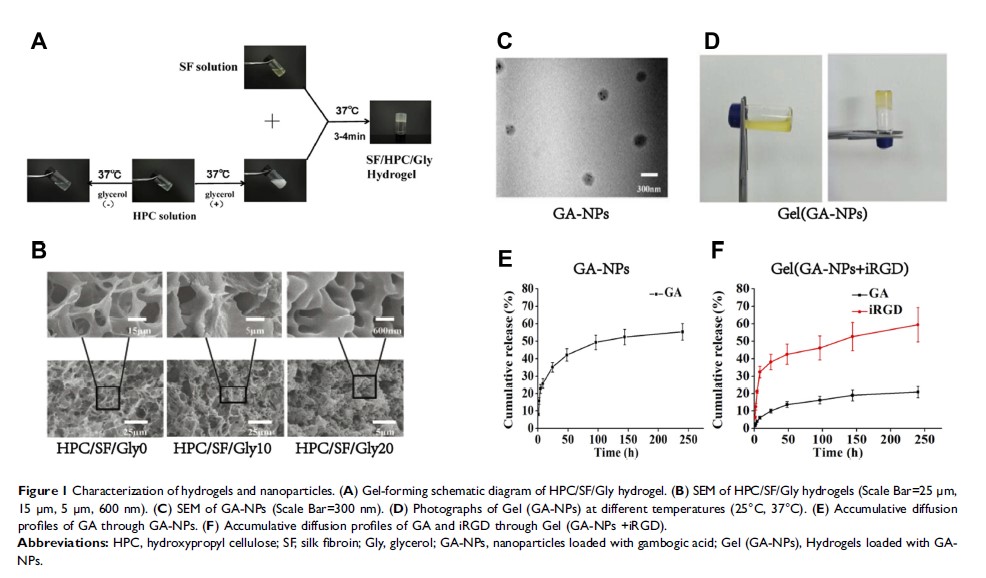
Introduction: Gambogic acid (GA) is proved to have anti-tumor effects on gastric cancer. Due to poor solubility, non-specific biological distribution, toxicity to normal tissues and short half-life, it is hard to be applied into the clinic. To overcome these issues, we developed a thermosensitive and injectable hydrogel composed of hydroxypropyl cellulose, silk fibroin and glycerol, with short gelling time, good compatibility and sustained release, and demonstrated that the hydrogel packaged with gambogic acid nanoparticles (GA-NPs) and tumor-penetrating peptide iRGD could improve the anti-tumor activity.
Methods: The Gelling time and micropore size of the hydrogels were regulated through different concentrations of glycerol. Controlled release characteristics of the hydrogels were evaluated with a real-time near-infrared fluorescence imaging system. Location of nanoparticles from different carriers was traced by confocal laser scanning microscopy. The in vivo antitumor activity of the hydrogels packaging GA-NPs and iRGD was evaluated by investigating tumor volume and tumor size.
Results: The thermo-sensitive properties of hydrogels were characterized by 3– 4 min, 37°C, when glycerol concentration was 20%. The hydrogels physically packaged with GA-NPs and iRGD showed higher fluorescence intensity than other groups. The in vivo study indicated that the co-administration of GA-NPs and iRGD by hydrogels had higher antitumor activity than the GA-loaded hydrogels and free GA combining with iRGD. Free GA group showed few antitumor effects. Compared with the control group, the body weight in other groups had no obvious change, and the count of leukocytes and hemoglobin was slightly decreased.
Discussion: The hydrogel constructed iRGD and GA-NPs exerted an effective anti-tumor effect possibly due to retention effect, local administration and continuous sustained release of iRGD promoting the penetration of nanoparticles into a deep part of tumors. The delivery system showed little systemic toxicity and would provide a promising strategy to improve anti-gastric cancer efficacy.
Keywords: gambogic acid, hydrogel, nanoparticles, iRGD, gastric cancer