110932
论文已发表
注册即可获取德孚的最新动态
IF 收录期刊
- 3.4 Breast Cancer (Dove Med Press)
- 3.2 Clin Epidemiol
- 2.6 Cancer Manag Res
- 2.9 Infect Drug Resist
- 3.7 Clin Interv Aging
- 5.1 Drug Des Dev Ther
- 3.1 Int J Chronic Obstr
- 6.6 Int J Nanomed
- 2.6 Int J Women's Health
- 2.9 Neuropsych Dis Treat
- 2.8 OncoTargets Ther
- 2.0 Patient Prefer Adher
- 2.2 Ther Clin Risk Manag
- 2.5 J Pain Res
- 3.0 Diabet Metab Synd Ob
- 3.2 Psychol Res Behav Ma
- 3.4 Nat Sci Sleep
- 1.8 Pharmgenomics Pers Med
- 2.0 Risk Manag Healthc Policy
- 4.1 J Inflamm Res
- 2.0 Int J Gen Med
- 3.4 J Hepatocell Carcinoma
- 3.0 J Asthma Allergy
- 2.2 Clin Cosmet Investig Dermatol
- 2.4 J Multidiscip Healthc

遏制 cyclinB1 可抑制肝癌细胞的增殖、侵袭和上皮间质转化,并增强对 TRAIL 诱导的细胞凋亡的敏感性
Authors Lv S, Ning H, Li Y, Wang J, Jia Q, Wen H
Received 29 July 2019
Accepted for publication 15 November 2019
Published 5 February 2020 Volume 2020:13 Pages 1119—1128
DOI https://doi.org/10.2147/OTT.S225202
Checked for plagiarism Yes
Review by Single-blind
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nicola Silvestris
Background: CyclinB1 is highly expressed in various tumor tissues and plays an important role in tumor progression. However, its role in hepatocellular carcinoma (HCC) remains unclear. Therefore, the aim of this study was to explore the role of cyclinB1 in the development and progression of HCC.
Methods: The expression of cyclinB1 was analyzed using the Gene Expression Profiling Interactive Analysis (GEPIA) database, and detected in HCC tissues and HCC cell lines through quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and Western blotting. CyclinB1-short hairpin RNA (Sh-cyclinB1) was transfected into HCC cells to knockdown cyclinB1, and the effect of cyclinB1 knockdown on HCC was examined via the MTT assay, colony formation assay, wound healing assay, scratch assay, cell cycle analysis in vitro, and xenograft model in nude mice. In addition, the role of cyclinB1 on tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis was measured using flow cytometry and Western blotting.
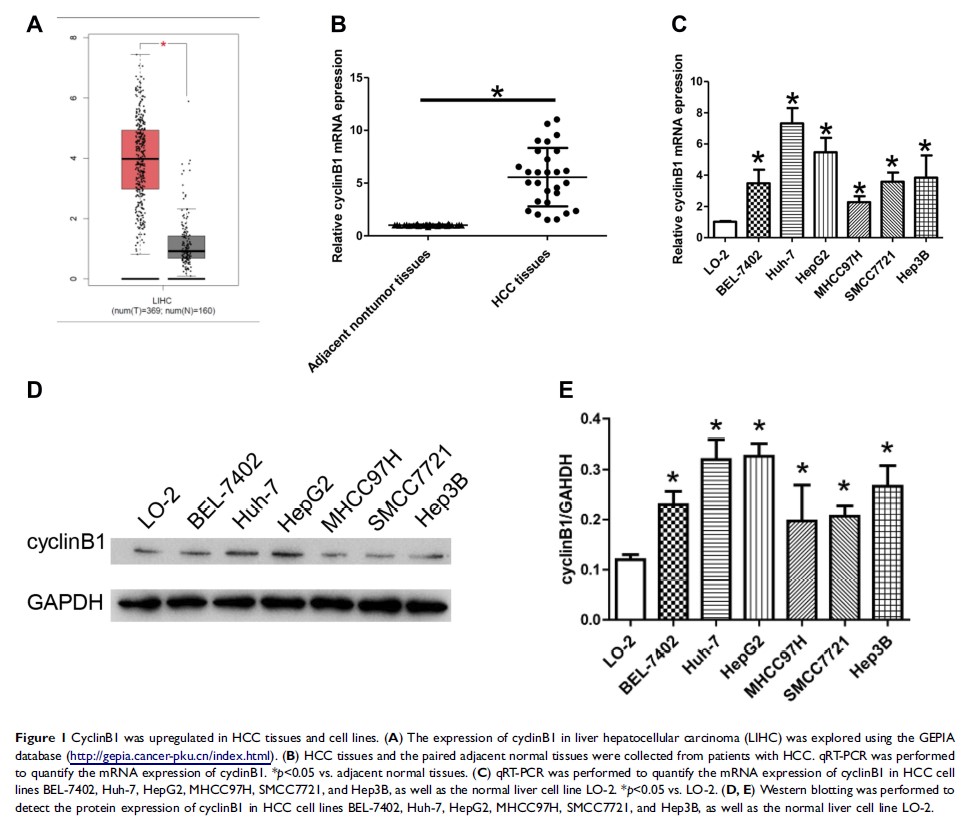
Results: The GEPIA database analysis showed that cyclinB1 was highly expressed in HCC tissues. The results of qRT-PCR and Western blotting proved that the expression of cyclinB1 was significantly increased in HCC tissues and cell lines. The data of the MTT assay, colony formation assay, and cell cycle analysis indicated that cyclinB1 knockdown inhibited the proliferation of HCC cells. In addition, cell migration, invasion, and epithelial mesenchymal transition were also impaired by cyclinB1 knockdown. Furthermore, the xenograft model in nude mice demonstrated that inhibition of cyclinB1 suppressed tumor growth and metastasis in vivo. Finally, the results of flow cytometry and Western blotting indicated that inhibition of cyclinB1 enhanced the sensitivity of HCC cells to TRAIL-induced apoptosis.
Conclusion: Overall, these data provide reasonable evidence that cyclinB1 may serve as a proto-oncogene during the progression of HCC.
Keywords: cyclinB1, proliferation, hepatocellular carcinoma, TRAIL